CHEMY.4M2.3WATER
WATER
WATER
Is a very important compound which is essential for the substance
of all living things.
Occurrence of Water
The water on the earth
occurs in three main states;
1. Solid
example:
Ice,
snow,
hail.
2. Liquid
example:
dew,
rain.
3.Vapor
example:
mist,
steam,
clouds.
• About 97% of all the
water on the Earth is salty water while only 13% is fresh water.
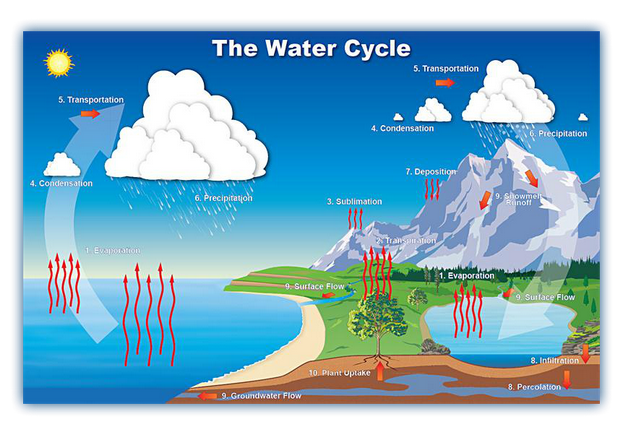
A cycle is a number of
change which come back to the starting point. Water is never lost but it is
continuously recycled around the globe in a system called water cycle.
The water cycle is made up of 4 main
stages:
1. Evaporation
2. Condensation
3. Precipitation
4. Collection
Physical properties of
water
1. It is colorless, odorless and tasteless.
2. It is only substance that occurs naturally in all the three
states of matter.
3. Pure water freezes at 0c°and boils at 100°c.
3. Pure water freezes at Oc and boils at 100°c.
4. It is universal solvent because it can dissolve more substance
than any other liquid.
5. It has a high specific heat index because it can absorb a lot
of heat before it begins to get hot.
6. It is miscible with many liquid for example ethanol.
Chemical properties of Water
1. Pure water is neutral
, it is neither acidic nor basic.
2. Cold water reacts with some metals such as potassium, sodium
and calcium to form metallic hydroxide and hydroxide and hydrogen gas.
Example:
Word equation
Cold
water + Potassium→Potassium hydroxide + Hydrogen
2H20(l)
+2K(S)→2KOH(S)+ H2(g)
Calcium+ Cold water→Calcium
hydroxide + Hydrogen
Ca(s) + 2H20
(l) →Ca(OH)2(s) + H2(g)
3. Steam (water vapor) reacts with some metals such as zinc,
Timonium and iron to produce metallic oxide and hydrogen gas.
WATER TREATMENT AND WATER PURIFICATION
Water treatment:
Is the process of making water usable for industrial, medical and
other purposes.
The aim is to remove existing contaminants in the water
Treatment process may be physical such as settling, chemical
eg.disinfection or biological
Water purification
Is the removal of contaminants from treated water to produce
drinking water, pure enough for human consumption. Substances that are removed
include bacteria, algae, fungi, minerals and human made chemical pollutants.
Domestic water purification
The common method used at
homes in purifying water
1. Boiling
2. Commercial filters and
3. Use of purifiers
1. Boiling:
During the method the
water is heated and left for sometimes before heating is stopped. This method
helps to kill disease - causing organism. The boiled water is then allowed to
cool before being used.
2.Commercial filter:
Use 80%, This filters
work by having the water pass through a charcoal element that purifies water
the filtered water is much clear than the original muddy water.
Role of:
Gravel: To trap any floating
substances.
Sand: To trap large
particles.
Charcoal: To kill some of harmful
bacteria.
Clean cloth: To filter the very tiny
particles.
3.Uses of purifiers:
Chemical purifiers are usually in liquid form. Are commended
amount of purifiers is put in a specific of water in a container. The water is
shake stirred) wool then left to set line for at least (20minutes) before it
can be sate for drinking. Example of Purifiers area Aqua guard, water guard.
TEST
FOR WATER
The presence or absence of water can be established by two
methods (regrets);
1. Copper (ii) sulphate solution
2. Cobalt chloride paper
1. Copper (ii) sulphate
White anhydrous copper (ii) sulphate turns blue on addition of
water. The reason is the formation of a new substance anhydrous copper (ii)
sulphate.
2. Cobalt chloride
Blue cobalt chloride
paper changes into pink when react with water.
NB:
Cobalt chloride test
is most
common substances than liquid (solution).
URBAN WATER TREATMENT
The water various
services before reaching their destiny is substance to see major stages namely;
1. Screening
2. Reservoir
3. Primary filtration
3. Primary filtration
4. Secondary filtration
5. Disinfection/ chlorination
6. Storage
1. Screening:
Is the stage once water is drawn from its
sources, the floating substances are removed.
2. Reservoir
The stage in which water is stored high up, so as it flows through
gravitation.
3. Primary filtration
Is a process in which large particles are removed, when they are
filtered through courses of sand.
Aluminum
sulphate is added to remove smaller particle how?
This is because Aluminum sulphate causes the impurities to chump
together and sink to the bottom of contain process is called Coagulation.
4. Secondary filtration
Is a process in which water is passing through
finer sand and thus causes removal of smallest particles.
5. Disinfection/chlorination:
Is a process in which chlorine added in a
moderate amount to kill harmful bacteria.
6.Stage
This is the final stage where by water is pure and safe enough to
be stored for use
Exercise
1. The diagram below
represent a simple water filter.
(a) Name the parts
labelled A to D.
A-Cloth
B-Charcoal
C- Sand
D-Gravel
(b) What is the importance of each part?
Gravel- To trap any floating
substance.
Sand- Is are removed large
particles.
Charcoal- Is to kill some of
harmful bacteria.
Cloth- To filter the very tiny
particles.
(c). What would be the disadvantages of using such as filter
to obtain drinking water?
- The
disadvantage is that It can cause disease.
IMPORTANCE OF WATER TREATMENT
Reason why water has to be treated:
1. Water that has not been treated may contain harmful and other
parasites that causes diarrhea , typhoid, cholera other illness.
2. Treated water is the best for using in laboratories to ensure
accurate result from experiments.
3. Treated water is suitable for using in factories to ensure the
actual products are Safe for consumption.
4. Treated water is more efficient to use for cleaning in
industries and domestic setting.
Conclusion: Untreated water lead to
usage of amount of certain substance such as soap for cleaning.





















0 on: "CHEMY.4M2.3WATER"